Stem Cell Treatments | NYC
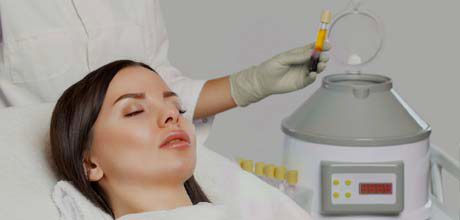
Stem Cell treatments are a new way to rejuvenate the skin and regrow hair – now available at Goldenberg Dermatology in Manhattan’s Upper East Side. Goldenberg Dermatology is pleased to announce that it is an exclusive dermatology partner of Predictive Biotech stem cell treatment, a cellular and regenerative medicine company. We were the first dermatology practice in New York City to offer the Stem Cell Aesthetics procedure.
In the last few decades, minimally-invasive technologies have changed the medical landscape. Today, doctors who perform cosmetic procedures have an increasingly sophisticated range of treatments at their disposal, which means that patients have greater possibilities to achieve their enhancement goals. For those wishing to combat the signs of aging, Stem Cell treatment can pave the way to better skin and thicker, fuller hair.
If you would like to learn more about how stem cell therapy can repair and restore your skin or hair, contact Goldenberg Dermatology to schedule a consultation at our Manhattan office today.